How Soap Works
How Soap Works
In recent days as the coronavirus outbreak escalates in the UK, we've received many queries about why the Government is advising us to wash our hands with soap. Some people are quite anxious that they are unable to buy sanitiser hand gels in the shops. From our conversations it's clear that very few people really understand how soap works, and they also do not appreciate that soap works far better than hand gels when it comes to tackling Covid-19. So we've written this article to give some more information.
Soap is fantastic! We love it, and we first began making it because we were fascinated by the chemistry that's involved and intrigued by the way that soap works. So please bear with us (there is a little bit of simple chemistry) and keep reading....
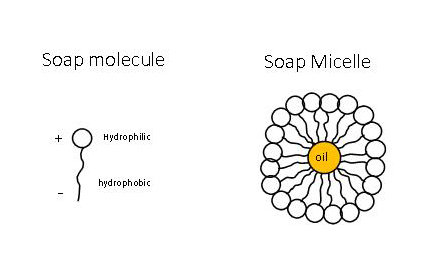 Soap is a chemical salt (it's made via saponification, which is the chemical reaction between an acid and an alkali) and each molecule has a head and a tail. The positively charged head is said to be hydrophilic (attracted to water) and the negatively charged tail is hydrophobic (repels water). When soap is mixed with water, the water becomes milky as the soap molecules disperse and form spherical structures called micelles. The soap molecule tails point inwards and the heads point outwards (see diagram). The tails of the soap molecule are attracted to grease/oil/dirt. So when you wash, the soap engulfs oil that's on the skin (the skin's natural sebum will have attracted dirt, bacteria etc) and suspends it inside the micelles so it can be rinsed away.
Soap is a chemical salt (it's made via saponification, which is the chemical reaction between an acid and an alkali) and each molecule has a head and a tail. The positively charged head is said to be hydrophilic (attracted to water) and the negatively charged tail is hydrophobic (repels water). When soap is mixed with water, the water becomes milky as the soap molecules disperse and form spherical structures called micelles. The soap molecule tails point inwards and the heads point outwards (see diagram). The tails of the soap molecule are attracted to grease/oil/dirt. So when you wash, the soap engulfs oil that's on the skin (the skin's natural sebum will have attracted dirt, bacteria etc) and suspends it inside the micelles so it can be rinsed away.
Handmade soap (like ours) is also naturally alkaline, so this means that each bar is more hygienic than many people think - few bugs can survive on the surface of a bar of soap.
How soap tackles coronavirus
So now you know how soap works, why is the Government and WHO (World Health Organisation) advising us to wash our hands for at least 20 seconds? It's so the soap can be in contact for long enough to effectively clean the skin, and engulf those oils and dirt. Another interesting fact is that covid-19 (unlike some other viruses) has a fatty outer membrane. So if you inadvertently get covid-19 on your skin and you then wash with soap, the soap molecules will hopefully engulf the virus so it can be rinsed away. Better still, research is now suggesting that soap damages the fatty outer layer of covid-19, and this can kill the virus.

Hand sanitiser gels (with at least 60% alcohol) work by killing the coronavirus that's on the skin. But you need to use plenty of gel and massage the skin for around 1 minute to give sufficient time for the alcohol to work. Unlike soap, hand sanitiser gels do not lift the virus from the skin so the problem is that many people do not use enough gel, or aren't working it into the hands for long enough for it to kill the virus. Something to bear in mind too, is that hand gels that contain high percentages of alcohol will dry out the skin with prolonged use.
Our soaps will not be so harsh to use, particularly now we're all washing our hands more frequently than before. We'd suggest that you try using some of our older Natroma soaps for hand washing - they have a reduced price because their fragrance has faded but will still lather beautifully.
We hope this information has helped to explain what happens when you wash your hands with soap. We hope you agree that regularly washing with soap and water for at least 20 seconds, is excellent advice during this covid-19 coronavirus outbreak. We are delighted to be manufacturing a product that is helping so many people and hope our Natroma handmade soaps help to keep people safe!

Really helpful information. Thank you Sarah & Paul.